
Harpoon has also developed a proprietary ProTriTAC™ platform, which applies a prodrug concept to its TriTAC platform to create a therapeutic T cell engager that remains inactive until it reaches the tumor.
HARPOON THERAPEUTICS TRIAL
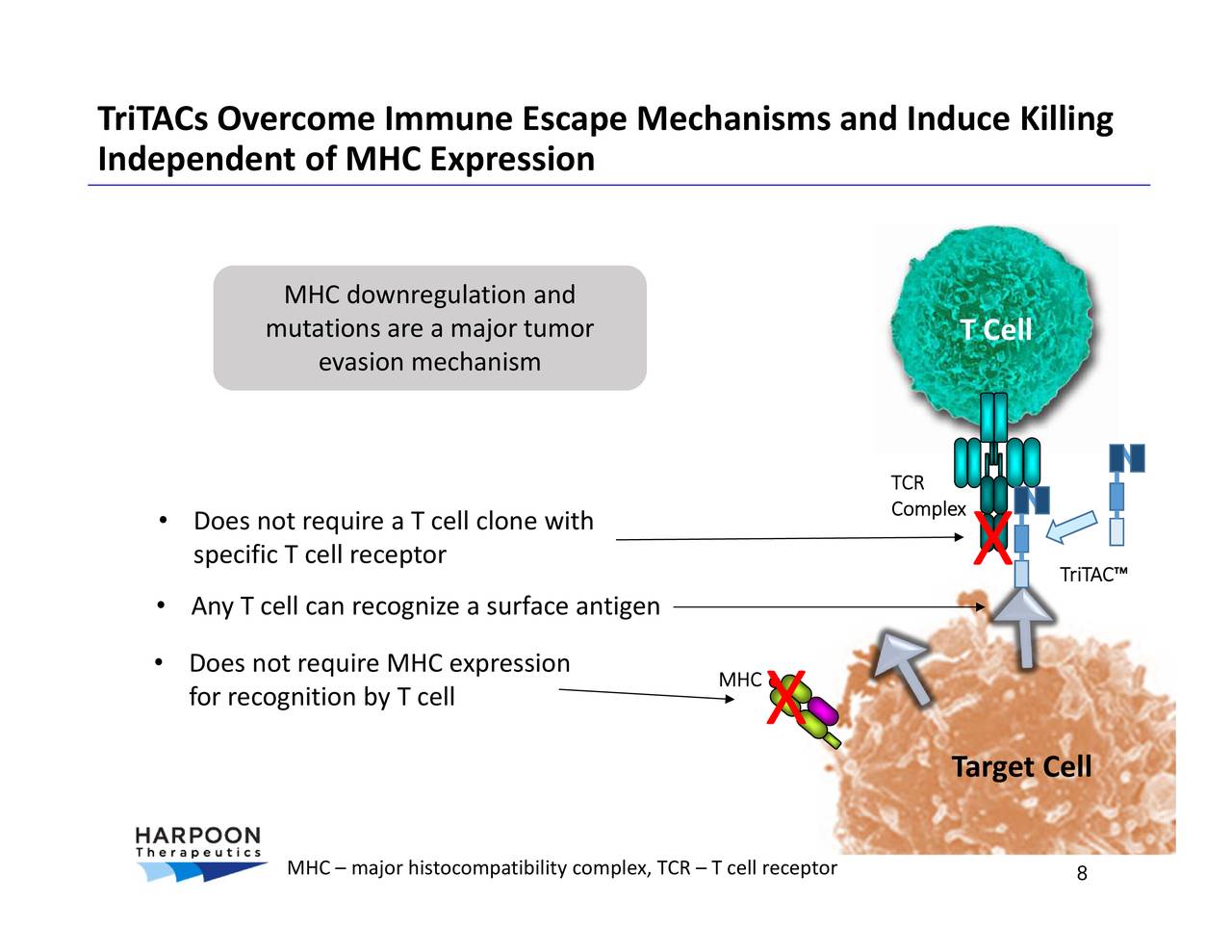
HPN328 targets DL元 and is in a Phase 1/2 trial for small cell lung cancer and other DL元-associated tumors. (HARP) reports results for the quarter ended September 2021. HPN217 targets BCMA and is in a Phase 1/2 trial for relapsed, refractory multiple myeloma. Wall Street expects a year-over-year increase in earnings on higher revenues when Harpoon Therapeutics, Inc. HPN536 targets mesothelin and is in a Phase 1/2a trial for cancers expressing mesothelin, initially focused on ovarian and pancreatic cancers. HPN424 targets PSMA and is in a Phase 1/2a trial for metastatic castration-resistant prostate cancer. Using its proprietary Tri-specific T cell Activating Construct (TriTAC ®) platform, Harpoon is developing a pipeline of novel TriTACs initially focused on the treatment of solid tumors and hematologic malignancies. T cell engagers are engineered proteins that direct a patient’s own T cells to kill target cells that express specific proteins, or antigens, carried by the target cells. Harpoon Therapeutics is a clinical-stage immunotherapy company developing a novel class of T cell engagers that harness the power of the body’s immune system to treat patients suffering from cancer and other diseases. A live webcast of the Canaccord presentation will be available from the Events and Presentations section of the company’s website at and will be archived there shortly after the event. A presentation at the Canaccord Genuity 41st Annual Growth Conference on Wednesday, Augat 10:30 a.m.A panel discussion titled “Heavenly (anti)Bodies” at the 2021 Wedbush PacGrow Healthcare Virtual Conference on Tuesday, Augat 1:10 p.m.(NASDAQ: HARP), a clinical-stage immunotherapy company developing a novel class of T cell engagers, today announced that Gerald McMahon, Ph.D., President and Chief Executive Officer, will participate in two upcoming virtual investor conferences: In the last 20 days, the company’s Stochastic K was 23.13 and its Stochastic D was recorded 13.40. CD3-targeted T cell engagers are potent anti-tumor therapies, but their development often requires management. The result represents downgrade in oppose to Raw Stochastic average for the period of the last 20 days, recording 43.45. Harpoon Therapeutics, South San Francisco, CA. The Company is engaged in the developing a T cell engager that harness the power of the bodyâs immune system to treat patients suffering from cancer and other diseases.
Securities and Exchange Commission, including under Risk Factors in Harpoon Therapeutics’ annual. is a clinical-stage immunotherapy company. in the period of last 50 days is set at 16.94. These and other factors that may cause Harpoon Therapeutics’ actual results to differ from those expressed or implied in the forward-looking statements in this press release are discussed in Harpoon Therapeutics’ filings with the U.S. Harpoon Therapeutics has increasingly invested its funds into research and development, jumping from a 15.1 million-dollar allocation in 2020 to 72.1 million in 2021. 03, 2021 (GLOBE NEWSWIRE) - Harpoon Therapeutics, Inc. Raw Stochastic average of Harpoon Therapeutics Inc.


 0 kommentar(er)
0 kommentar(er)
